Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- NEWS FEATURE
- 20 December 2024

Stem cells head to the clinic: treatments for cancer, diabetes and Parkinson’s disease could soon be here
- Alison Abbott 0
Alison Abbott is a writer based in Munich, Germany.
You can also search for this author in PubMed Google Scholar
A team at Skåne University Hospital in Lund, Sweden, prepares a needle to transplant cells into a person’s brain to treat Parkinson’s. Credit: Åsa Sjöström for Nature
Andrew Cassy had spent his working life in a telecommunications research department until a diagnosis of Parkinson’s disease in 2010 pushed him into early retirement. Curious about his illness, which he came to think of as an engineering problem, he decided to volunteer for clinical trials.
“I had time, something of value that I could give to the process of understanding the disease and finding good treatments,” he says.
In 2024, he was accepted into a radical trial. That October, surgeons in Lund, Sweden, placed neurons that were derived from human embryonic stem (ES) cells into his brain. The hope is that they will eventually replace some of his damaged tissue.

Stem cells reverse woman’s diabetes — a world first
The study is one of more than 100 clinical trials exploring the potential of stem cells to replace or supplement tissues in debilitating or life-threatening diseases, including cancer, diabetes , epilepsy, heart failure and some eye diseases . It’s a different approach from the unapproved therapies peddled by many shady clinics, which use types of stem cell that do not turn into new tissue.
All the trials are small and focus mainly on safety. And there are still substantial challenges, including defining which cells will be most fit for which purposes and working out how to bypass the need for immunosuppressant drugs that stop the body from rejecting the cells but increase the risk of infections.
Still, the flurry of clinical studies marks a turning point for stem-cell therapies. Following decades of intense research that has at times triggered ethical and political controversy , the safety and potential of stem cells for tissue regeneration is now being widely tested. “The rate of progress has been remarkable,” says stem-cell specialist Martin Pera at the Jackson Laboratory in Bar Harbor, Maine. “It’s just 26 years since we first learnt to culture human stem cells in flasks.”
Researchers expect some stem-cell therapies to enter the clinic soon. Treatments for some conditions, they say, could become part of general medicine in five to ten years.
Finding a source
Cassy’s symptoms began with a small, persistent tremor in his fingers when he was just 44. The characteristic motor symptoms of Parkinson’s are driven by the degeneration of dopamine-producing neurons called A9 cells in the brain’s substantia nigra. Drugs that replace the missing dopamine are effective, but have side effects including uncontrolled movements and impulsive behaviours. And as the disease progresses, the drugs’ efficacy wanes and the side effects worsen.
The idea of replacing the degenerated dopaminergic cells has a long history. During development, pluripotent ES cells, which have the potential to become many cell types, turn into the specialized cells of the brain, heart, lungs and so on. Theoretically, transplanted stem cells could repair any damaged tissue.
Parkinson’s lent itself to testing that theory. The first transplant of such cells took place in Sweden in 1987 using neurons from the developing brains of fetuses from terminated pregnancies, the only source of immature, or progenitor, neural cells at the time. Since then, more than 400 people with Parkinson’s have received such a transplant — with mixed results . Many people saw no benefit at all, or had debilitating side effects. But others improved so much that they no longer needed to take dopaminergic drugs.
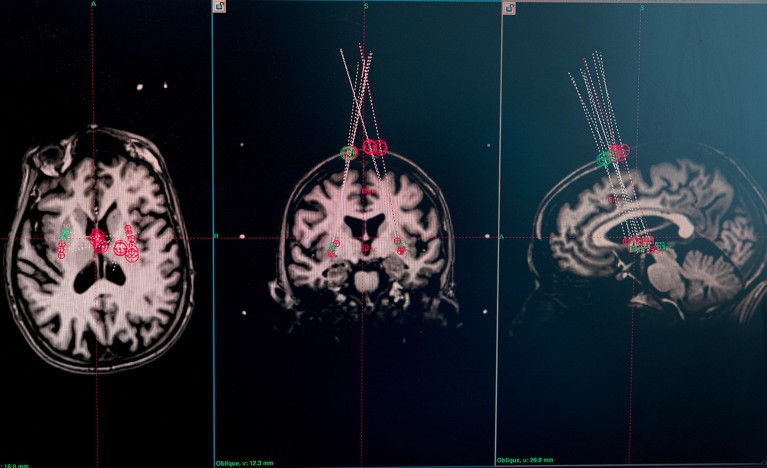
Brain MRI scans of a trial participant are used to plan where the needle will deliver the cells. Credit: Åsa Sjöström for Nature
“Overall, the studies showed us that the approach can work, sometimes transformatively,” says neurologist Roger Barker at the University of Cambridge, UK. “But we needed a more-reliable source material.”
Fetal brain tissue cannot be standardized and might also be contaminated with progenitors that are destined to mature into the wrong sort of cells. On top of this, some people have ethical or religious objections to the use of this material . And in any case, notes Barker, it has often been hard to find enough material to go ahead with an operation to transplant the cells.
Prospects for regenerative stem-cell therapy improved when it became possible to derive specialized cells from more controllable sources, particularly human ES cells and, later, induced pluripotent stem (iPS) cells , which are created by reprogramming adult cells to revert to an immature state. Today, large numbers of specialized cells can be reliably produced at a quality and purity high enough for the clinic.
Stem-cell researcher Agnete Kirkeby at the University of Copenhagen and her colleagues have surveyed the landscape of regenerative-stem-cell clinical trials worldwide and, as of December 2024, they had identified 116 trials approved or completed across a range of diseases 1 . Around half use human ES cells as the starting material. The other studies use iPS cells, either off the shelf or generated from the skin cells or blood of individual people to treat their own conditions. Twelve of the trials attempt to treat Parkinson’s disease using dopamine-producing cells derived from stem cells.
Promise for Parkinson’s
The trial that Cassy is enrolled in, which Barker co-leads, and another more-advanced trial run by BlueRock Therapeutics, a biotechnology firm based in Cambridge, Massachusetts, gave participants A9 progenitor cells derived from human ES cells. The BlueRock trial has reported preliminary results for its 12 participants. Two years in, the treatment has proved safe and shown hints of efficacy in those receiving the higher of two doses. So far, no Parkinson’s trial has reported uncontrolled movement side effects such as those seen with dopaminergic drugs and in some trials that used fetal tissue.

The race to supercharge cancer-fighting T cells
Compared with other organs, such as the heart, pancreas and kidneys, the brain has proved to be one of the most straightforward organs to treat with stem cells. One advantage is that the brain is largely protected from the body’s immune system, which seeks out and destroys foreign tissue. Participants in Parkinson’s trials receive immunosuppressants for only a year to cover the period when the blood–brain barrier is healing from surgery. Participants in trials for other organs typically receive the drugs for the rest of their lives.
And the brain is accommodating. The A9 cells usually reside in the substantia nigra and send projections out to the putamen, in the forebrain, where they release dopamine. But neurosurgeons often place the progenitor cells directly in the putamen because it’s easier to get at surgically. The brain’s ability to adapt to fetal tissue and to cells transplanted into the ‘wrong’ site is “pretty clever”, says Barker.
Just as remarkable, he says, is a study of epilepsy in which transplanted cells derived from human ES cells integrate into the correct neural circuits in the brain. In the clinical trial, run by the biotechnology company Neurona Therapeutics based in San Francisco, California, surgeons transplanted immature versions of a type of brain cell called interneurons into the brains of ten people with a form of epilepsy that could not be controlled by drugs. Before receiving this treatment, the participants’ seizures were so frequent and debilitating that they could not live independently.

A rat brain with transplanted stem-cell-derived cells is put on ice and cut into slices in preparation for analysis. These cells were tested in rats before an ongoing clinical trial in people with Parkinson’s disease was approved. Credit: Åsa Sjöström for Nature
One year after the transplant, the frequency of severe seizures in the first two participants had dropped to almost zero, an effect that has been maintained for two years. Most of the other participants have had pronounced reductions in seizure frequency. There were no significant side effects and no cognitive damage, the company reports . Last June, the US Food and Drug Administration awarded the therapy a fast-track status to expedite the process that leads to regulatory approval.
“The outcomes for patients were strikingly similar even though procedures were carried out at different sites around the country,” says Arnold Kriegstein at the University of California, San Francisco, who is a co-founder of Neurona Therapeutics. “It is very robust.”
Like the brain, the eye is well-protected from the body’s immune system. Kirkeby and her colleagues identified 29 clinical trials for ocular diseases, particularly for types of age-related macular degeneration. Other organs don’t have the same immune privilege, yet are responsible for some of the most burdensome diseases, including heart failure as well as type 1 diabetes, which is caused by the destruction of insulin-producing islet cells in the pancreas.
Beyond the brain and eyes
Progress has been slower for other conditions. But positive early results from a trial run by the drug company Vertex Pharmaceuticals based in Boston, Massachusetts, have spawned a rush of optimism for diabetes. Stem-cell biologist Douglas Melton and his colleagues developed the first functional islet cells from a human ES cell line in 2014 at Harvard University in Cambridge 2 . Now at Vertex, he is leading a trial of people with particularly serious forms of the disease, using proprietary islet cells generated by similar methods. The cells do their job wherever they are placed in the body, in this case the liver. According to the company, 9 of the 12 participants who received the full dose no longer need to inject insulin, and another two were able to reduce their dose.
“I was surprised and delighted that it worked so well,” says Melton, who moved into this field in the 1990s, when his baby son was diagnosed with type 1 diabetes. “And especially glad to see the potential it has for patients.”
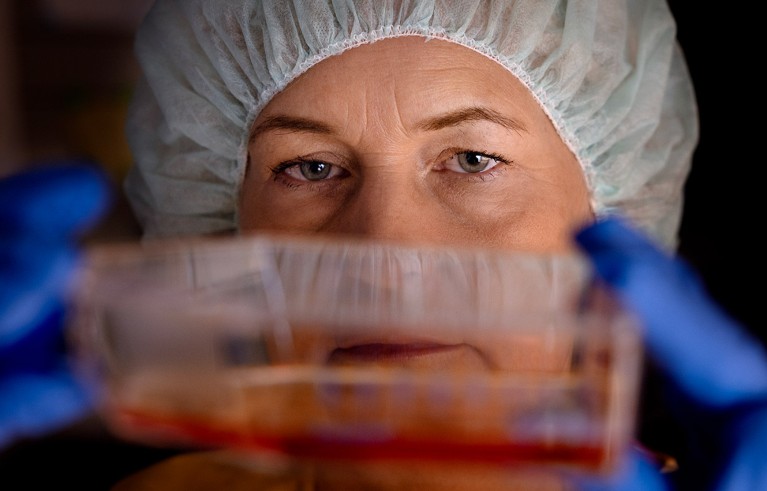
The laboratory of Malin Parmar at Lund University, Sweden, developed stem-cell-derived replacement cells for an ongoing trial that is attempting to use stem-cell therapy to replace damaged tissue in people with Parkinson’s disease. Credit: Åsa Sjöström for Nature
The heart has proved particularly vexing for regenerative medicine. It’s a large and complex pump made up of different cell types, and any damage must be fixed in situ . Stem-cell scientist Christine Mummery at Leiden University in the Netherlands, was one of the first to generate beating heart-muscle cells 3 , or cardiomyocytes, from human ES cells in 2002. But, she quickly realized how challenging it would be to bring to the clinic, particularly when she saw a deeply scarred and fatty heart removed during a transplant surgery. “I thought: we won’t be able to fix that any time soon.” She changed her research direction to disease modelling. But with roughly 64 million people worldwide with heart failure, Mummery says she values the persistence of those who haven’t given up.
Enjoying our latest content? Login or create an account to continue
- Access the most recent journalism from Nature's award-winning team
- Explore the latest features & opinion covering groundbreaking research
Nature 637 , 18-20 (2025)
doi: https://doi.org/10.1038/d41586-024-04160-0
Kirkeby, A. et al. Cell Stem Cell (in the press).
Paglucia, F. W. et al. Cell 159 , 428–439 (2014).
Article PubMed Google Scholar
Mummery, C. et al. J. Anat. 200 , 233–240 (2002).
Takahashi, K. & Yamanaka, S. Cell 126 , 663–676 (2006).
Download references
Reprints and permissions
Related Articles
- Therapeutics
- Cell biology
Nucleosome fibre topology guides transcription factor binding to enhancers
Article 18 DEC 24

Spatial transcriptomic clocks reveal cell proximity effects in brain ageing
Timely TGFβ signalling inhibition induces notochord
Lipid-delivery system could treat life-threatening pregnancy complication
News & Views 11 DEC 24
Next-generation snakebite therapies could reduce death toll
Outlook 21 NOV 24
Cephalopod-inspired jetting devices for gastrointestinal drug delivery
Article 20 NOV 24
Engineered extrachromosomal oncogene amplifications promote tumorigenesis
A lipid made by tumour cells reprograms immune cells
Neutralizing GDF-15 can overcome anti-PD-1 and anti-PD-L1 resistance in solid tumours
Article 11 DEC 24
Editorial Director, Nature Research Journals
Job Title: Editorial Director, Health and Applied Sciences, Nature Portfolio (m/f/d) Location: New York or Berlin Reports to: VP of Research Journa...
New York City, New York (US)
Springer Nature Ltd
Chief Editor, Nature Sensors
Job Title: Chief Editor, Nature Sensors (m/f/d) Locations: New York or Berlin Application Deadline: January 5, 2025 About Springer Nature Group S...
Assistant Professor of Biomedical Informatics
The Department of Biomedical Informatics at Harvard Medical School invites applications for a tenure-track Assistant Professor position focused on ...
Boston, Massachusetts (US)
Harvard Medical School
Assistant Professor in Biodiversity Studies Using eDNA
Join Umeå Plant Science Centre as Assistant Professor in Biodiversity Studies and explore the links between biodiversity and plant physiology
Umeå (Kommun), Västerbotten (SE)
Umeå University

New Positions open for the laser science center in Peking University
The Beijing Laser Accelerator Innovation Center at Peking University invites applications for Applied Physics faculty.
Beijing, China
Peking University (PKU)
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Neural stem cell transplantation shows promise for treating chronic spinal cord injury
A Phase I clinical trial led by researchers at University of California San Diego School of Medicine has demonstrated the long-term safety and feasibility of neural stem cell transplantation for treating chronic spinal cord injuries. These devastating injuries often result in partial or full paralysis and are currently incurable. The study, which followed four patients with chronic spinal cord injuries for five years, found that two patients showed durable evidence of neurological improvement after treatment with neural stem cell implantation, including increased motor and sensory scores, and improved electromyography (EMG) activity. Some patients also showed improvement in pain scores.
Neural stem cell transplantation is an emerging treatment for various neurological disorders and injuries that works by implanting human-derived stem cells into damaged or diseased areas of the nervous system. Because these neural stem cells are derived from human cells, this treatment approach has the potential to regenerate damaged tissue while integrating seamlessly into the existing nervous system.
The study found that all four patients tolerated the treatment well, and while the current study was only designed to assess safety and tolerability, the results suggest that neural stem cell transplantation may have therapeutic potential for treating chronic spinal cord injuries. Following these promising results, the researchers now hope to initiate a phase II clinical trial to assess the treatment's efficacy.
The study, published in the December 17 edition of Cell Reports Medicine , was led by Joseph Ciacci, M.D., a professor in the Department of Neurological Surgery at UC San Diego School of Medicine and neurosurgeon at UC San Diego Health, and Joel Martin, M.D., who was a neurological surgery resident physician at UC San Diego at the time the study was completed and is now a neurosurgeon at Orlando Health. The research was supported by the California Institute of Regenerative Medicine (CIRM) UC San Diego Alpha Stem Cell Clinic and the Sanford Stem Cell Clinical Center within the Sanford Stem Cell Institute.
- Medical Topics
- Nervous System
- Brain-Computer Interfaces
- K-12 Education
- Neuroscience
- Stem cell treatments
- Embryonic stem cell
- Adult stem cell
- Spinal cord
- Artificial neural network
- Bone marrow transplant
- Somatic cell
Story Source:
Materials provided by University of California - San Diego . Original written by Miles Martin. Note: Content may be edited for style and length.
Journal Reference :
- Joel R. Martin, Daniel Cleary, Mickey E. Abraham, Michelle Mendoza, Betty Cabrera, Catriona Jamieson, Martin Marsala, Joseph D. Ciacci. Long-term clinical and safety outcomes from a single-site phase 1 study of neural stem cell transplantation for chronic thoracic spinal cord injury . Cell Reports Medicine , 2024; 5 (12): 101841 DOI: 10.1016/j.xcrm.2024.101841
Cite This Page :
Explore More
- Laser-Based Artificial Neuron: Lightning Speed
- Large Hadron Collider Regularly Makes Magic
- New, Improved Flu-Vaccine Construct
- Influencing When Infants Speak
- Icy Planetesimals of the Early Solar System
- Unique Asteroid-Comet Hybrid
- 50 Hidden Relatives of the First Pterosaur
- Novel Hydrothermal Vents On Arctic Ocean Floor
- Water Reservoirs, Rare Magmas On Ancient Mars
- Origins of Lunar Water: New Findings
Trending Topics
Strange & offbeat.
- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
Stanford Medicine study hints at ways to generate new neurons in old brains
The researchers’ finding suggests the possibility of designing pharmaceutical or genetic therapies to turn on new neuron production in old or injured brains.
October 2, 2024 - By Gordy Slack

Stanford Medicine scientists demonstrated that knocking out glucose transporter genes had an activating and proliferative effect on neural stem cells in mice. olga_demina - stock.adobe.com
Most neurons in the human brain last a lifetime, and for good reason. Intricate, long-term information is preserved in the complex structural relationships between their synapses. To lose the neurons would be to lose that critical information — that is, to forget.
Intriguingly, some new neurons are still produced in the adult brain by a population of cells called neural stem cells. As brains age, however, they become less and less adept at making these new neurons, a trend that can have devastating neurological consequences, not just for memory, but also for degenerative brain diseases such as Alzheimer’s and Parkinson’s and for recovery from stroke or other brain injury.
A new Stanford Medicine study , published Oct. 2 in Nature , sheds hopeful new light on how and why neural stem cells, the cells behind the generation of new neurons in the adult brain, become less active as brains age. The research also suggests some intriguing next steps in addressing old neural stem cell passivity — or even stimulating neurogenesis, the production on new neurons, in younger brains in need of repair — by targeting newly identified pathways that could reactivate the stem cells.
Anne Brunet , PhD, professor of genetics, and her team used CRISPR platforms, molecular tools that allow scientists to precisely edit the genetic code of living cells, to conduct a genome-wide search for genes that, when knocked out, increase the activation of neural stem cells in cultured samples from old mice, but not from young ones.
“We first found 300 genes that had this ability — which is a lot,” emphasized Brunet, the Michele and Timothy Barakett Endowed Professor. After narrowing the candidates down to 10, “One in particular caught our attention,” Brunet said. “It was the gene for the glucose transporter known as the GLUT4 protein, suggesting that elevated glucose levels in and around old neural stem cells could be keeping those cells inactive.”

Anne Brunet
Dynamic brains
There are parts of the brain, such as the hippocampus and the olfactory bulb, where many neurons have shorter lives, where they regularly expire and may be replaced by new ones, said Tyson Ruetz, PhD, a formal post-doctoral scholar in Brunet’s lab and the lead author of the Nature paper. “In these more dynamic parts of the brain, at least in young and healthy brains,” he said, “new neurons are constantly being born and the more transient neurons are replaced by new ones.”
Ruetz, now the scientific advisor and co-founder of ReneuBio, developed a way to test the newly identified genetic pathways in vivo, “where the results really count,” Brunet said.
Ruetz took advantage of the distance between the part of the brain where the neural stem cells are activated, the subventricular zone, and the place the new cells proliferate and migrate to, the olfactory bulb, which is many millimeters away in a mouse brain. By knocking out the glucose transporter genes in the former, waiting several weeks, then counting the number of new neurons in the olfactory bulb, the team demonstrated that knocking out the gene indeed had an activating and proliferative effect on neural stem cells, leading to a significant increase in new neuron production in living mice. With the top intervention, they observed a more than twofold increase in newborn neurons in old mice.
“It’s allowing us to observe three key functions of the neural stem cells,” Ruetz said. “First, we can tell they are proliferating. Second, we can see that they’re migrating to the olfactory bulb, where they’re supposed to be. And third, we can see they are forming new neurons in that site.”
The same technique could also be applied to studies of brain damage, Ruetz said. “Neural stem cells in the subventricular zone are also in the business of repairing brain tissue damage from stroke or traumatic brain injury.”
‘A hopeful finding’
The glucose transporter connection “is a hopeful finding,” Brunet said. For one, it suggests not only the possibility of designing pharmaceutical or genetic therapies to turn on new neuron growth in old or injured brains, but also the possibility of developing simpler behavioral interventions, such as a low carbohydrate diet that might adjust the amount of glucose taken up by old neural stem cells.
The researchers found other provocative pathways worthy of follow-up studies. Genes relating to primary cilia, parts of some brain cells that play a critical role in sensing and processing signals such as growth factors and neurotransmitters, also are associated with neural stem cell activation. This finding reassured the team that their methodology was effective, partly because unrelated previous work had already discovered associations between cilia organization and neural stem cell function. It is also exciting because the association with the new leads about glucose transmission could point toward alternative avenues of treatment that might engage both pathways, Brunet said.
“There might be interesting crosstalk between the primary cilia — and their ability to influence stem cell quiescence, metabolism and function — and what we found in terms of glucose metabolism,” she said.
“The next step,” Brunet continued, “is to look more closely at what glucose restriction, as opposed to knocking out genes for glucose transport, does in living animals.”
The work was supported by the National Institutes of Health (grants P01AG036695 and R01AG056290), a Wu Tsai Neurosciences Institute Big Ideas in Neurosciences Grant (the Stanford Brain Rejuvenation Project ) and a Larry L. Hillblom Foundation Postdoctoral Fellowship.
- Gordy Slack Gordy Slack is a freelance writer.
About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
The majestic cell
How the smallest units of life determine our health


- News Releases
Research alert: Neural stem cell transplantation shows promise for treating chronic spinal cord injury
University of California - San Diego
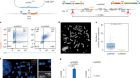
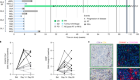
This graphical abstract illustrates the study design. Notable innovations include the use of a “floating cannula” to deliver stem cells without suspension of respiration, utilizing diffuse tensor imaging (DTI) to enable both qualitative and quantitative assessment of the spinal cord, and utilizing electromyography (EMG) to reveal evidence of neurological improvements.
Credit: Martin et al./Cell Reports Medicine
A Phase I clinical trial led by researchers at University of California San Diego School of Medicine has demonstrated the long-term safety and feasibility of neural stem cell transplantation for treating chronic spinal cord injuries. These devastating injuries often result in partial or full paralysis and are currently incurable. The study, which followed four patients with chronic spinal cord injuries for five years, found that two patients showed durable evidence of neurological improvement after treatment with neural stem cell implantation, including increased motor and sensory scores, and improved electromyography (EMG) activity. Some patients also showed improvement in pain scores.
Neural stem cell transplantation is an emerging treatment for various neurological disorders and injuries that works by implanting human-derived stem cells into damaged or diseased areas of the nervous system. Because these neural stem cells are derived from human cells, this treatment approach has the potential to regenerate damaged tissue while integrating seamlessly into the existing nervous system.
The study found that all four patients tolerated the treatment well, and while the current study was only designed to assess safety and tolerability, the results suggest that neural stem cell transplantation may have therapeutic potential for treating chronic spinal cord injuries. Following these promising results, the researchers now hope to initiate a phase II clinical trial to assess the treatment’s efficacy.
The study, published in the December 17 edition of Cell Reports Medicine , was led by Joseph Ciacci, M.D., a professor in the Department of Neurological Surgery at UC San Diego School of Medicine and neurosurgeon at UC San Diego Health, and Joel Martin, M.D., who was a neurological surgery resident physician at UC San Diego at the time the study was completed and is now a neurosurgeon at Orlando Health. The research was supported by the California Institute of Regenerative Medicine (CIRM) UC San Diego Alpha Stem Cell Clinic and the Sanford Stem Cell Clinical Center within the Sanford Stem Cell Institute.
Cell Reports Medicine
10.1016/j.xcrm.2024.101841
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.

IMAGES
COMMENTS
Nov 27, 2024 · Stem cells articles from across Nature Portfolio. ... Latest Research and Reviews. ... This paper develops two approaches for multiplexing cortical organoids and SCanSNP, a method for deconvolving ...
1 day ago · Oct. 30, 2024 — New research into the long-term dynamics of transplanted stem cells in a patient's body explains how age affects stem cell survival and immune diversity, offering insights that ...
2 days ago · As stem cells are the source of all tissues, understanding their properties helps in our understanding of the healthy and diseased body's development and homeostasis. Latest Research and Reviews
Aug 30, 2024 · Stem cell research encounters varied regulations across countries, posing challenges for international collaboration. Some nations impose stringent restrictions on embryonic stem cell research, while others adopt more permissive policies [28]. Recent changes in international regulations offer insight into how different regions navigate stem ...
20 hours ago · The laboratory of Malin Parmar at Lund University, Sweden, developed stem-cell-derived replacement cells for an ongoing trial that is attempting to use stem-cell therapy to replace damaged tissue ...
3 days ago · Using neural stem cells could help treat spinal injuries once thought to be untreatable -- a new phase 1 trial has determined this approach is safe. A Phase I clinical trial led by researchers at ...
Aug 30, 2024 · Stem cell transplantation has emerged as a promising avenue in regenerative medicine, potentially facilitating tissue repair in degenerative diseases and injuries. This review comprehensively examines recent developments and challenges in stem cell transplantation. It explores the identification and isolation of various stem cell types, including embryonic, induced pluripotent, and adult stem ...
Oct 2, 2024 · A new Stanford Medicine study, published Oct. 2 in Nature, sheds hopeful new light on how and why neural stem cells, the cells behind the generation of new neurons in the adult brain, become less active as brains age. The research also suggests some intriguing next steps in addressing old neural stem cell passivity — or even stimulating ...
Mar 1, 2023 · The general characteristics of CSCs have been widely summarized by several groups. Briefly, CSCs have the ability to self-renew in asymmetric (generating a new stem cell and a differentiated cell) and symmetric (generating two identical stem cells) patterns and differentiate into other cells to generate heterogenous cell types [3]. Apart from ...
3 days ago · Neural stem cell transplantation is an emerging treatment for various neurological disorders and injuries that works by implanting human-derived stem cells into damaged or diseased areas of the ...